CBD Snacks, Beverages May Present Issues for Retailers
by
By Robin Mather
A marijuana and hemp derivative called CBD is starting to show up in all kinds of snacks, from bites to bars to cookies to coffee shots to energy drinks. Many questions swirl around these CBD-enhanced products that retailers may wish to consider.
CBD is short for cannabidiol; its qualities are reputedly therapeutic, but proponents say CBD doesn’t make its user euphoric. THC, perhaps marijuana’s most famous component, may also have therapeutic properties, but it is psychoactive – it will make its users high. Both compounds may be detected in drug tests.
Both compounds bind to receptors in the human body – there are dozens, in the brain, gut and elsewhere. Both compounds are said to relieve pain, nausea, and anxiety; CBD may also help its users who suffer from other health conditions, including migraines and depression, while THC may help those with glaucoma, low appetite, and PTSD.
In states where marijuana is legal for both recreational and medical use, retailers may be able to sell CBD products. Some states have passed laws that make CBD for medical use legal. CBD is generally recognized as safe (GRAS) when made from hemp.
Checking the legality of CBD products in a retailer’s state is easy. But federal, state and local laws often conflict.
In January, the U.S. Senators from Oregon, Ron Wyden and Jeff Markley, sent a letter to the U.S. Food and Drug Administration, asking the FDA to update its regulations to allow the interstate sale of food products containing CBD. Though the senators asked for a response within 30 days, there’s no timeline for the FDA to act. FDA Commissioner Scott Gottlieb has reiterated the FDA’s position that CBD is a drug and therefore illegal to add to food or health products without FDA approval. The FDA has sent warning letters to some companies making health claims for CBD.
Ronie Schmelz is an attorney in Los Angeles, California, who is also licensed in New York. She regularly counsels companies that want to create CBD products. “Here in L.A., you can buy CBD shots to add to your coffee, or order a CBD-enhanced martini,” she says. “But that doesn’t mean it’s legal. We’re starting to see a little bit more enforcement activity. [Her clients] haven’t seen any warning letters, but we have started seeing local and federal inspections.”
Many CBD products are for skin and hair care, she notes, but more and more companies are producing CBD edibles. “CBD is ingestible, but we don’t know how much we can take, or should take,” she says.
Lack of Science, Lack of Regulation
Because marijuana is still a schedule-1 drug, Schmelz notes, little research has been done in the U.S. to prove CBD’s efficacy. While the newest Farm Bill made the cultivation and transportation of hemp legal, it hasn’t changed marijuana’s status. “There has also been no modification in the Federal Food, Drug and Cosmetic Act concerning hemp or CBD,” she says.
Scientists have noted that few comprehensive clinical studies exist in the U.S. to determine how CBD affects humans. Canada, which legalized medical marijuana in 2001, and Israel, which legalized some uses of medical marijuana in the early 1990s, have done the most research on the effects of THC and CBD, says industry observer Michael Klein.
Klein, the Chief Executive Officer of cannabismd.com, describes his website as “the only non-advocacy platform for the potential benefits of medical marijuana,” and says he wants “to empower consumers to make choices that are right for them.”
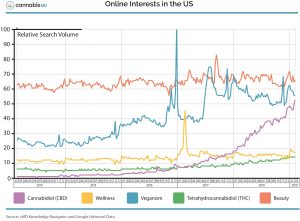 While state and federal authorities tussle over legality, Klein says, consumer interest in CBD products has skyrocketed. “CBD is now one of the most searched terms on the internet,” he says. “It used to be that ‘food’ was the most searched terms. But now, if you put ‘food’ against wellness, beauty, and CBD, just in the last nine months, CBD has surpassed food.”
While state and federal authorities tussle over legality, Klein says, consumer interest in CBD products has skyrocketed. “CBD is now one of the most searched terms on the internet,” he says. “It used to be that ‘food’ was the most searched terms. But now, if you put ‘food’ against wellness, beauty, and CBD, just in the last nine months, CBD has surpassed food.”
This, he says, is a monumental shift. “You’re seeing consumers connecting on CBD faster than the media can cover it. They’ve had positive experiences with it, and they want to share their experience with others.”
How Much CBD is too Much?
Among those questions, says Dr. Rob Streisfeld, a naturopathic doctor, is how much CBD is safe, and how much is overkill.
Streisfeld is the founder of Beyond Brands, an agency that promotes “conscious brands, with a focus on plant-based foods, products and awareness.” He’s also the author of two books, including “The Cannabis Conundrum.”
Streisfeld says he expects the CBD snack industry to grow. “It [CBD] seems to have an extremely safe profile, and with increased awareness comes the desire to incorporate it into foods, beverages and other consumer products. Bars, cookies and chocolate are going to have CBD added. Beverages in general are going to be a huge category,” he says.
Adding CBD to foods makes sense, he adds. “Many of the receptors for CBD are in the digestive system, making food and beverages a desirable method of delivery,” Streisfeld says.
But there are a couple of hitches. “CBD comes in two forms: a broad spectrum, which includes other compounds from the plant, and an isolate, which separates out CBD from all other compounds. The challenge in the industry is to decide which form to use. Research shows that CBD has more effectiveness when the other compounds are present. Are we giving up functionality for legality if we choose the isolate?”
The other problem, in Streisfeld’s view, is market saturation. “At what point will saturation not only decrease the value of using CBD in formulation?” he says. “At what level does the industry defeat itself?” Like the rush to add probiotics to products, he says, “it may be a race to the bottom.” Until the FDA clarifies its position, he says, “this is going to be a challenging space, and even more so because of the eyes of the feds.”




